Boiling Point of Ester and Carboxylic Acid
Fats and oils are esters as are many important fragrances and flavors. Carboxylic acids are more polar than alcohols because there are two oxygen atoms present in a carboxylic acid molecule.

Physical Properties Of Carboxylic Acids Youtube
For more information about fats and oils see.
. 12 rows Boiling Point Polar Rank most to least Name Brief Explanation. Common odours can be found in. They even have higher boiling points than other compounds with weaker or fewer dipoles like amines alcohols phenols aldehydes ketones esters amides and isosteric.
Hydrolysis of ester gives carboxylic acid and. Table 211 lists the solubilities and boiling points of some esters. Esters are common solvents.
Aldehydes ketones carboxylic acids esters and amides. 1 lists the physical properties of some common esters. Only carboxylic acids can undergo intermolecular hydrogen bonding.
Carboxylic acids can make strong hydrogen bonds but esters cannot. View Carboxylic acids and esters recapdocx from CHEMISTRY 44 at Read Foundation College Samahni. Esters have a general formula of RCOOR.
Carboxylic acid any of a class of organic compounds in which a carbon C atom is bonded to an oxygen O atom by a double. Ethyl acetate is used to extract organic solutes from aqueous solutionsfor. The boiling points of carboxylic acids increases as the molecules get bigger.
Physical properties of carboxylic acids and esters. Included in the larger group of carbonyl compounds are the narrower families. Or carboxylic acids cannot be reduced to aldehydes by hydride reducing agents because aldehydes are more.
The flammability of alcohols decrease as the size and. What force is responsible for the difference in boiling point between carboxylic acids. View FILE_20220901_223645_Week 8Carboxyclic acid esters amides amines_2135932579pdf from CHEM 1120 at Illinois Eastern Community Clg.
Chapter 5 Carboxylic Acids and Esters 19 Name Molecular weight Boiling point Solubility in water Pentane 72 gmol 35C Insoluble Diethyl ether 74 gmol 35C Insoluble Butanal 72 gmol 76C. A fourth bond links the. Carboxylic acids have even higher boiling points then alkanes and alcohols.
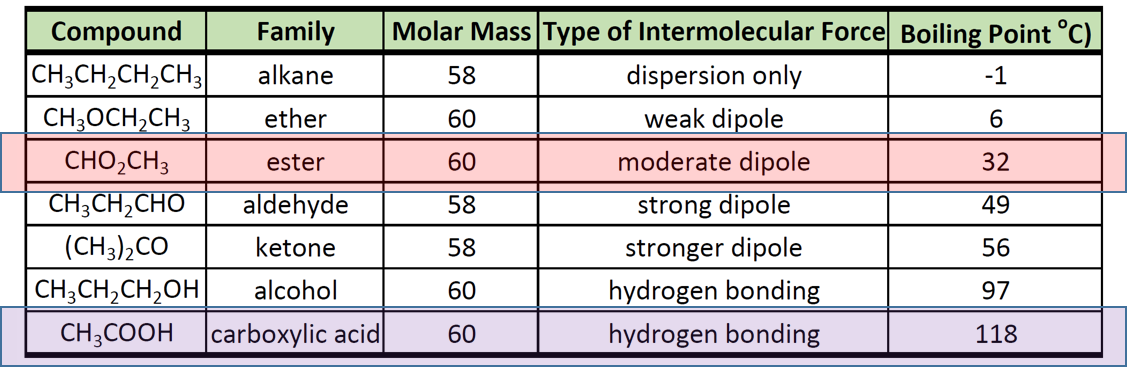
Perhaps it is surprising that the amide appears to be the most polar. 12 Comparison of Boiling Points Compound Molar Mass Boiling Point O CH. Hydrolysis of ester gives carboxylic acid and.
A dimer of acetic acid have higher boiling points than alcohols ketones and aldehydes of similar mass. Boiling points of esters are lower than that of carboxylic. Since 2010 diesel fuel may contain up to 7 vol fatty acid methyl ester FAME in Europe to meet biofuels.
Carboxylic acids have higher boiling points than. The carbonyl group utilizes two of. An ester is derived from a carboxylic acid and an alcohol.

Solubility And Boiling Point Flashcards Quizlet
Ch105 Chapter 9 Organic Compounds Of Oxygen Chemistry

Carboxylic Acid Have Higher Boiling Points Than Aldehydes Ketones And Even Alcohol Of Youtube

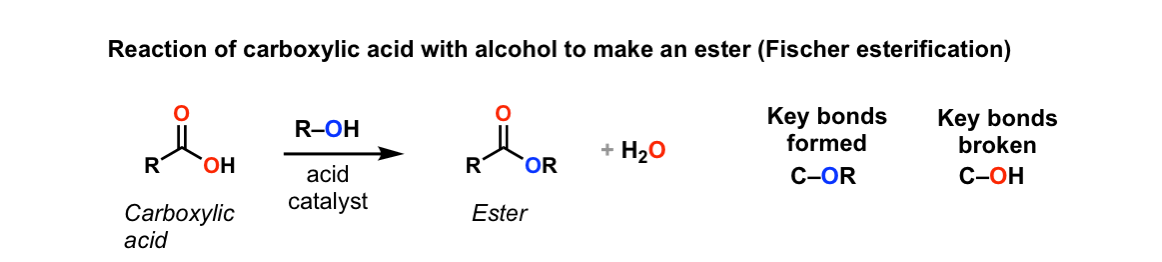
Conversion Of Carboxylic Acids To Esters Using Acid And Alcohols Fischer Esterification Master Organic Chemistry
No comments for "Boiling Point of Ester and Carboxylic Acid"
Post a Comment